Abstract : The composite phase change energy storage material was prepared with sodium acetate trihydrate as the energy storage unit and the epoxy resin as the carrier. It shows a strong stability and energy storage effect at the melting point temperature. By adding expanded graphite having high thermal conductivity and porous adsorption to the composite phase change energy storage material, the thermal conductivity and sealing performance can be further improved. The results show that when the mass fraction of sodium acetate trihydrate is 60% and that of expanded graphite is 5%, the phase change energy of the phase change energy storage material is 148.5 J/g, the thermal conductivity is 0.891 W/(m°C), and the stability is good. .
[Chinese Packaging News] Phase change energy storage materials (PCMs) have been widely used in solar energy utilization, electric power peaking, waste heat utilization, cross-seasonal due to their advantages of high energy storage density and approximately isothermal storage (release) of heat. Heat storage and cold storage, food preservation, building insulation, agriculture and other fields.
Sodium acetate trihydrate (CH3COONa·3H2O) is a promising phase change energy storage material with a high energy storage density, a heat of fusion of 264 J/g, and a melting point of 58°C, compared to organic phase change energy storage materials. The thermal conductivity is high, but as a phase change energy storage material there is a liquid leakage problem in the solid-liquid phase transition process. There are two main methods currently used to solve this problem:
Firstly, the phase change energy storage material is wrapped in a microcapsule with a polymer material as a shell to form a microcapsule phase change energy storage material. The material prepared by this method has good sealing properties, but the process is more complicated; second, the phase change is performed. The energy storage material and the polymer material are blended and melted, and the phase change material is wrapped in the network structure of the polymer material. Wang Shouxu et al. used a solidified epoxy resin as a carrier and a Na2HPO4·12H2O phase change material as an energy storage unit. The composite phase change energy storage material produced had excellent sealing performance and was simple and convenient to process and shape. However, the thermal conductivity of the composite phase change energy storage material with epoxy resin as the carrier will be significantly reduced, resulting in a decrease in the energy conversion efficiency of the material. In order to improve the thermal conductivity of phase change energy storage materials, currently used methods are mainly through the addition of high thermal conductivity materials in phase change energy storage materials, such as metal materials Al, Cu, Ag, metal oxides A12O3, MgO, BeO, and others. Carbon black, graphite, SiN, AIN, etc. Weilong Wang et al. used A1N to fill the composite phase change energy storage materials of polyethylene glycol (PEG) and silicon dioxide (SiO2). When the A1N content was 30%, the thermal conductivity of the composite phase change energy storage material was 0.766 W/( m°C).
This article will use sodium acetate trihydrate as an energy storage unit, solidified epoxy resin as a carrier, and at the same time fill with expanded graphite as a thermally conductive filler to prepare a composite phase change energy storage material.
l Preparation and testing of composite phase change energy storage materials 1.1 Reagents and instrument reagents: CH3COONa·3H2O (analytical grade), epoxy resin (epoxy value 0.44), expanded graphite (expansion factor 220).
Instruments: Pyris Diamond Differential Thermal Analyzer (DSC, Perkin Elmer, USA); PYRIS1 Thermogravimetric Analyzer (TGA, Perkin Elmer, USA); JSM-5610LV Scanning Electron Microscopy (SEM, JEOL) );YBF-3 Thermal Conductivity Tester (Hangzhou Dahua Instrument Manufacturing Company); QM-1SP2 Planetary Ball Mill (Nanjing University Instrument Factory); PL-S Electronic Balance (Metterle Shanghai Co., Ltd.); Thermocouple , template number.
1.2 Preparation method of composite phase change energy storage material A certain amount of sodium acetate trihydrate and the added material are weighed in a ball mill tank according to the mass ratio, and the ball mass medium is added to the ball mill tank at a mass ratio of 4:1 to grind the grinding media. In 40 minutes, a uniform ultrafine powder was obtained. Then, a certain amount of expanded graphite was weighed into the ball mill tank and the ball milling was continued for 10 minutes so that the powder and the expanded graphite were fully adsorbed and uniformly mixed. In addition, a certain amount of epoxy resin and a curing agent (mass ratio of epoxy resin to curing agent 1: (0.8-1)) were weighed in a beaker and stirred evenly. The mixed epoxy resin is then added to the ball mill tank and the powder adsorbed by the expanded graphite is subjected to ball milling and mixing for 15 minutes to allow the epoxy resin to embed the powder. Then, the mixed ingredients were transferred to a template of different shapes for molding and cured at 20° C. for 48 hours to obtain a composite phase change energy storage material.
1.3 Structural Characterization and Performance Testing 1.3.1 Observation of Morphology of Composite Phase Change Energy Storage Materials Using liquid nitrogen to perform brittle fracture of the sample, the cross section of the sample was characterized by scanning electron microscopy, and the dispersion of sodium acetate, graphite, etc. in the sample system was observed. Happening.
1.3.2 Fusion heat of composite phase change energy storage material A certain amount of sample was weighed and mounted on an aluminum plate for DSC scanning. The temperature rise range was 40-160°C and the heating rate was 10°C/min. Use the data analysis software of the instrument system to analyze the phase change heat and melting point crystallization temperature of the sample.
1.3.3 Thermal stability of composite phase change energy storage materials To test the thermal stability of composite phase change energy storage materials, it was heated from room temperature (20°C) to 70°C with a thermogravimetric analyzer (TGA) at a heating rate of 10 °C / min, and then kept at a constant temperature of 70 °C 30min, test its quality changes, in order to determine the stability of the phase change energy storage unit in the carrier.
1.3.4 Thermal conductivity of composite phase change materials Thermal conductivity (thermal conductivity) is a physical quantity that reflects the thermal conductivity of a material. The steady-state slab method was used to measure the thermal conductivity of the material. The device for measuring the thermal conductivity was shown in Figure 1.
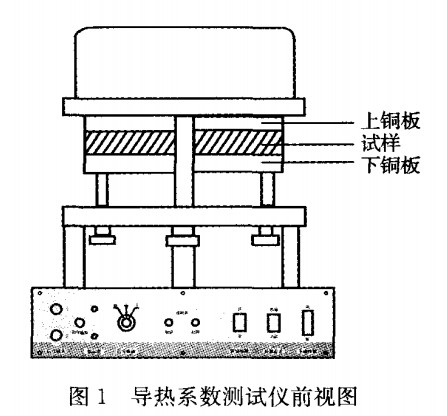
The upper copper plate in Fig. 1 is provided with a flat panel heater to provide a heat source. When heated, the bottom surface of the upper copper plate directly transfers heat to the sample. At the same time, the sample transfers the absorbed heat through the lower surface of the sample and continues to the copper plate. When the heat is transmitted from the sample Equal to the heat transferred from the sample, the sample is in a stable thermal conduction state. At this time, the temperature of the surface of the upper and lower copper plates of the sample is a stable value, and the upper plate is T1, and the lower plate is T2. According to the Fourier equation, the thermal conductivity of the sample can be deduced:

In the formula: λ - thermal conductivity of a sample, R - radius of a sample, height of a sample of h, mass of permanent companion of m, specific heat capacity of c - copper block, RP and hP are the radius and thickness of the lower copper plate, respectively.
1.3.5 Determination of the energy conversion rate of the composite phase change material Take samples prepared in different proportions, drill a hole in the sample using a fine drill, and insert a thermocouple probe into the hole to test the internal temperature of the sample. The sample was placed in a constant temperature water bath and heated at 80°C for 30 minutes. The temperature per minute of each sample was recorded by a computer data acquisition system. The samples were taken from a constant temperature water bath and cooled in a 40°C environment while the temperature per minute of each sample was still recorded by a computer data acquisition system. According to the recorded time and temperature, the energy exchange rate curve of the sample is plotted.
2 Experimental results and analysis
2.1 Morphological analysis of composite phase change energy storage materials Scanning electron microscopy can be used to observe the dispersion of components in composite phase change materials. Figure 2 shows SEM images of PCMs without and with 3% expanded graphite when the sodium acetate trihydrate content is 60%. As can be seen from Figure 2, the energy storage unit sodium acetate trihydrate is uniformly distributed in the epoxy resin carrier with fine particles of about 1 μm, and the carrier directly encapsulates the functional unit with good sealability. The SEM images of expanded graphite PCMs with mass fraction of 3% can be seen that the sodium acetate trihydrate particles are large, a part of which is first adsorbed by the expanded graphite, and then their adsorption communities are embedded by the epoxy resin carrier at the same time. .

2.2 Phase change analysis of composite phase change energy storage material Figure 3 shows the DSC curve of composite phase change energy storage material and single component.

From Figure 3, it can be seen that the DSC curve of the pure epoxy resin in the range of 4O to 160 °C is basically a straight line, there is no obvious endothermic and exothermic peaks, indicating that the cured epoxy resin has the conditions of this study The stable thermal performance will not affect the heat absorption and heat release of the energy storage unit material and can be used as a carrier for a stable composite phase change energy storage material. In addition, from the DSC curve of pure sodium acetate trihydrate, a strong phase transition endothermic peak and a dehydration endothermic peak began to appear at 58.1°C and 121.5°C, respectively, and the latent heat of phase change at the temperature of 58.1°C was 242.7. J/g, similar to literature values. This shows that sodium acetate trihydrate is an ideal high energy storage phase change material. At the same time, compared with the DSC curve of pure sodium acetate trihydrate, the composite phase change energy storage material shows the maximum absorption peak at 60.2°C, and the position has a tendency to move toward high temperature. This may be due to the fact that the carrier of the composite energy storage material forms a sealed system, which changes the phase change environment of the phase change material. When the temperature rises, the internal pressure of the system will increase accordingly (volumetric effects of solid-liquid conversion, expansion of the sealing gas). And other factors), thereby affecting the position of the phase change point. This result is corroborated with the depletion of the dehydration peak of the composite phase change energy storage material at 121.5°C.
